Peptides: The Body’s Tiny Messengers and the Future of Medicine
Peptides are fascinating molecules that form the foundation of life’s complex biochemistry. They are often called the body’s “signaling molecules” because they act as tiny, powerful messengers—directing cells and tissues to perform specific actions such as healing a wound, regulating hormones, or maintaining metabolic balance.
This article explains what peptides are, how they form, and why they are becoming one of the most exciting frontiers in medicine and biotechnology.
🧬 What Exactly Is a Peptide?
At the simplest level, a peptide is a biological compound made of two or more amino acids linked together. Amino acids are the basic building blocks of proteins and essential to nearly every function in the human body.
The Peptide Bond
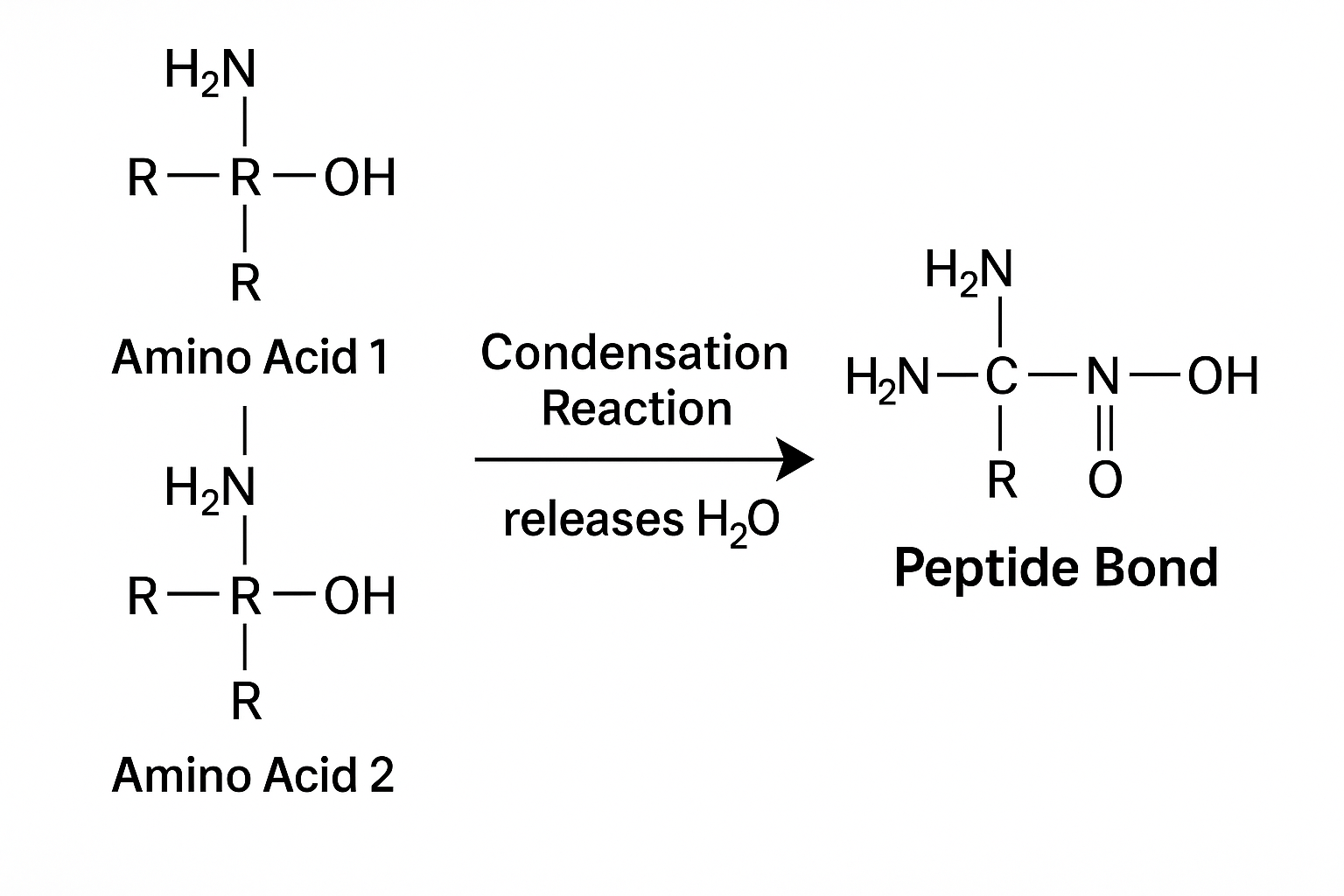
The defining feature of a peptide is the peptide bond (also known as an amide bond):
- Formation: The carboxyl group (–COOH) of one amino acid reacts with the amino group (–NH₂) of another.
- Condensation Reaction: This releases a molecule of water (H₂O).
- Outcome: A stable CO–NH covalent bond is formed, linking the amino acids together.
The word peptide comes from the Greek term péssēin (πἐσσειν), meaning “to digest.” Thousands of peptides occur naturally in the body and participate in nearly every physiological process.
🏭 How Are Peptides Formed?
Peptides arise through two broad routes:
1. Natural biological formation
2. Synthetic chemical production
🔬 Ribosomal vs. Nonribosomal Peptide Synthesis (Diagram)
To understand where peptides come from, it helps to see how living organisms create them.
There are two major natural systems:
- Ribosomal synthesis (used by humans, animals, and all higher organisms)
- Nonribosomal synthesis (used by bacteria, fungi, and plants)
Diagram: Ribosomal vs. Nonribosomal Peptide Synthesis
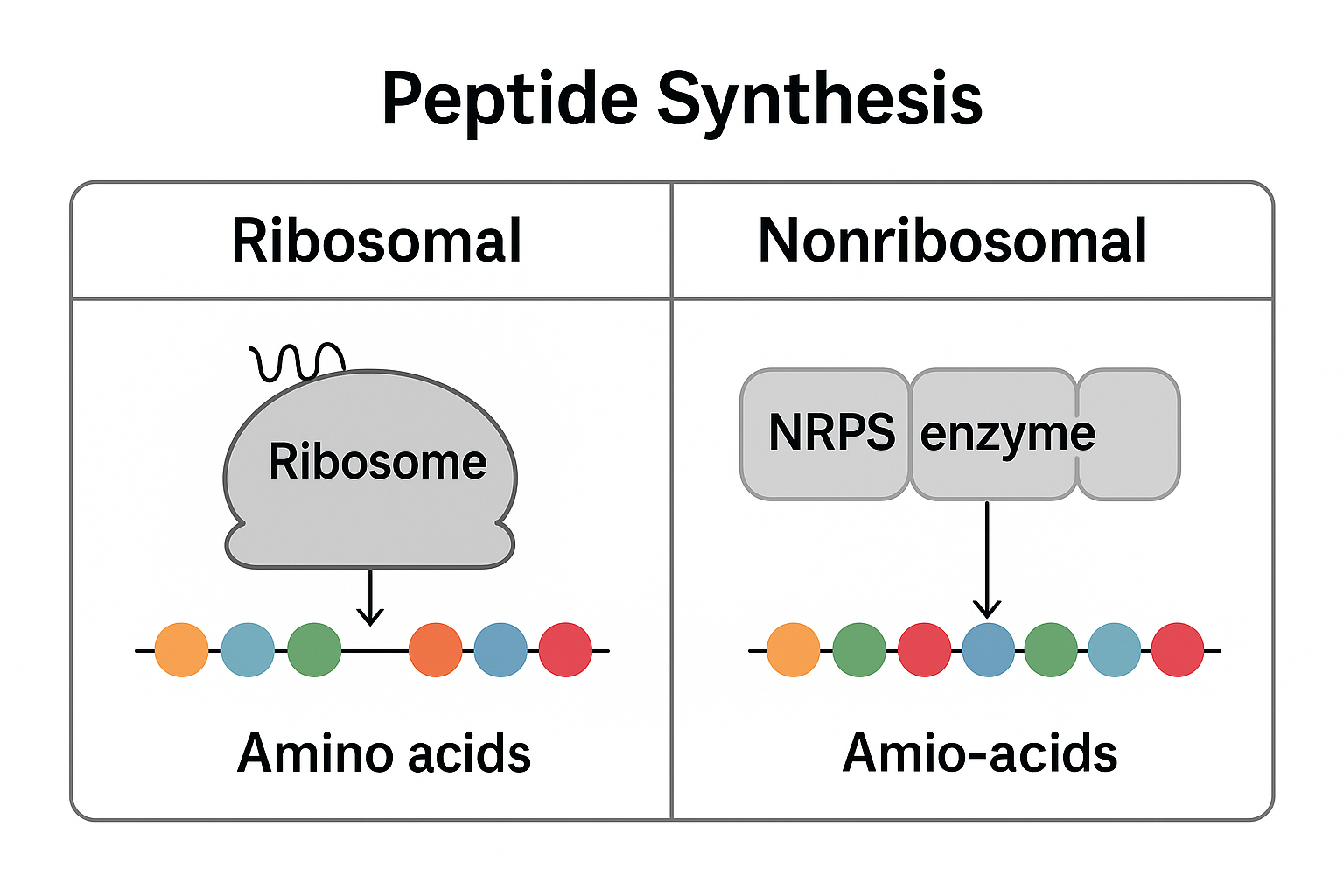
Ribosomal Peptides
Produced through translation, the same process used to create proteins.
Examples: insulin, glucagon, growth-related peptides, opioid peptides
Nonribosomal Peptides (NRPs)
Produced by NRPS enzymes without using mRNA.
Examples: glutathione, penicillin backbone compounds, certain antimicrobial peptides
🧪 Synthetic Peptide Formation
Modern chemistry allows scientists to build peptides artificially for research and therapeutics.
Solid Phase Peptide Synthesis (SPPS)
The dominant modern method.
Peptides are built step-by-step on a solid resin bead.
Liquid Phase Peptide Synthesis
An older technique still used for specialty chemistries.
Historical milestones:
- 1901: First synthetic peptide
- 1953: Full chemical synthesis of oxytocin
📏 Visual Comparison: Peptides vs. Proteins (Diagram)
Length is the simplest way to distinguish peptides from proteins.
Classification Table
| Classification | Amino Acid Count | Description / Example |
| Dipeptide | 2 | Smallest possible peptide. |
| Tripeptide | 3 | Three amino acids. |
| Oligopeptides | 2–10 | Short signaling peptides. |
| Polypeptides | ~10–40/50 | Longer chains, may not fold. |
| Proteins | > ~50 | Fully folded functional structures. |
Peptides vs Proteins
Figure 2.
Peptides are short amino acid chains; proteins are long, folded molecules.
The Fuzzy Line
The 50-amino-acid guideline is not strict.
- Amyloid-beta (40 aa) is often treated as a protein.
- Insulin (51 aa) is often referred to as a peptide hormone.
Function and structure matter more than length.
🧭 Additional Ways to Classify Peptides
Milk Peptides
Bioactive fragments released from casein and whey during digestion or fermentation.
Peptones
Mixtures of small peptides used as nutrient media for microbial growth.
Peptide Fragments
Pieces of larger proteins produced via enzymatic breakdown.
Peptide discovery is accelerating rapidly, offering enormous potential in regenerative medicine, targeted therapies, metabolic optimization, and next-generation pharmaceuticals.